
Free Radicals - A Clear And Simple Explanation
Posted on 20 Feb, 2019

What is a free radical?
A free radical is a molecule with a missing electron, which makes it highly unstable and very reactive.
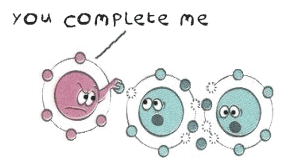 How does a missing electron make it reactive?
How does a missing electron make it reactive?
An atom’s chemical behaviour is determined by the number of electrons in its outermost shell. When the outermost shell is full, the atom is stable and tends not to engage in chemical reactions. But when the outermost shell is not full, it will try and stabilize itself by either gaining or losing an electron. Sometimes it will share its electrons by bonding with another atom that is also looking to complete its outer shell.
 And why is this bad?
And why is this bad?
A molecule with a missing electron will go on a rampage to rob other molecules of their electron. Once it succeeds in doing that, it leaves its victim short of an electron, making a free radical of it. As the cycle continues, it can trigger a chain reaction where up to 6.023 x 1021 billion molecules react per second, and this can wreak havoc on living tissue.
It would not be so bad if free radicals merely caused cells to die after acquiring the missing electron. What happens is that it alters the DNA in the cell, and it becomes mutated. The altered cell will grow abnormally, and reproduce abnormally, which creates the seed for diseases like cancer, Alzheimer's and Parkinson's. Free radicals also bring about an accumulation of damaged cells within your inner arterial walls, causing it to thicken. As the plaque in your arteries increase, so do your chances of cardiovascular disease.

Where do free radicals come from?
Free radicals are sometimes formed as a by product of the body’s natural human physiological processes. In the way a car produces exhaust fumes, the body, through the chemical processes of digestion and metabolism, can produce free radicals.
Free radicals also exists in the environment and can be a risk to our health. Environmental pollutants like smog, cigarette smoke and car exhaust fumes all contain free radical molecules. Consumption of alcohol and exposure to UV rays also lead to the forming of free radicals.
How are free radicals formed internally?
To generate energy, our body utilizes oxygen to metabolize food. Oxygen, because it carries two unpaired electrons in its outer shell, is very reactive to anything it comes in contact with. During the conversion process, most of the oxygen combines with hydrogen to form water. Some pair up with carbon to produce carbon dioxide. But it’s not a perfect system. Between 2 to 5 percent of the oxygen we take in leads to the forming of free radicals via electron escape.
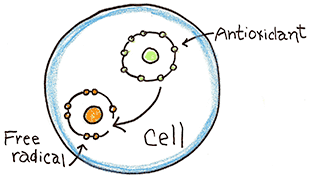 How do you counter free radicals?
How do you counter free radicals?
Your body has an inherent ability to defend against free radicals by producing three substances; catalase , glutathione and superoxide dismutase. These substances are known as antioxidants. Antioxidants have extra electrons in their outershell which they can donate to free radicals to neutralize them.
 The importance of free radicals
The importance of free radicals
Believe it or not, free radicals, bad as they are, have a role to play in our survival. Our immune system marks damaged tissue and foreign invaders with free radicals. This is so it knows what cells to expel from our body. The death and disposal of cells brings about the birth of new ones. And this process of regeneration is vital to the body’s health.
Photo Credits
Books For Better Health

by Angela Liddon

by Tess Masters

by David Wolfe

by Robin Jeep

 7 Habits Of Truly Happy People
7 Habits Of Truly Happy People 8 Ways To Speed Up Muscle Recovery
8 Ways To Speed Up Muscle Recovery Free Radicals - A Clear And Simple Explanation
Free Radicals - A Clear And Simple Explanation Should You Stop Exercising? A look into exercise and free radicals, and its impact on your health.
Should You Stop Exercising? A look into exercise and free radicals, and its impact on your health.










